



Bovine tuberculosis: A continuing threat to cattle around the world
An introduction to bovine tuberculosis (bTB)Bovine tuberculosis (bTB) is a chronic infectious disease that primarily affects cattle, but it can also affect humans, goats, pigs, cats, dogs, deer, badgers, and other mammals.
bTB is caused by slow-growing, facultative intracellular bacteria of the Mycobacterium tuberculosis complex [1]. Most bTB infections are caused by Mycobacterium bovis, but bTB can also be caused by Mycobacterium caprae and, less frequently, by Mycobacterium tuberculosis.
Prevalence, Economic Impact, and Challenges
bTB is one of the diseases listed by the World Organisation for Animal Health (WOAH), and it must be reported if detected [1].
Over 50 million cattle are infected worldwide, and it is estimated that economic losses due to bTB add up to about $3 billion annually. bTB costs the UK alone about USD $145 million each year.
Infection rates may be as high as 50% in some areas in Africa, although prevalence of the disease varies by region. bTB is rare in Europe, Canada, and the US. Outbreaks typically occur in areas where livestock is adjacent to wildlife populations. The disease is endemic in Central and South America, and it is significantly more prevalent in dairy cattle [2].
Transmission and risks
bTB is primarily a respiratory disease that affects lymph glands in the throat and lungs of infected cattle.
Evidence strongly suggests that most transmission occurs through direct contact with infectious aerosols from infected animals [3], although bTB can be spread through contact with nasal discharge, saliva, milk, and the placenta of an infected animal [4]. It can also be transmitted indirectly in unsanitary environments or through ingestion of contaminated food or water.
While bTB is considered a zoonotic disease, humans who ingest unpasteurized milk or come into contact with infected tissue while processing meat can be infected. bTB is thus a risk to farmers, their families, farm workers, and the public.
In addition to harming animal health, bTB can reduce profits and undo years of effort to improve genetic production outcomes. Movement of cattle is thus a major concern with implications for trade, although import and export regulations around positive bTB cases vary by country. The movement of herds that test positive for bTB will generally be restricted until all animals test negative for the disease.
The UK and Ireland have made costly efforts to eradicate bTB since the 1950s, but they have not been successful due to the complex, multispecies nature of the disease and contributions by wildlife to the pathogen load in the environment. However, both countries have significantly reduced the prevalence of bTB within their borders.
Successful bTB eradication programs have utilized a multifaceted approach that includes [1]:
- Postmortem meat inspections to search for tubercles in the lungs, lymph nodes, intestines, liver, spleen, pleura, and peritoneum to identify infected animals and herds
- Intensive surveillance, including on-farm visits
- Systematic testing of individual cattle
- Removal of infected animals and animals that have come into contact with them
- Adequate local legislation
- Effective control of cattle movement
- Individual animal identification
- Effective tracing
Symptoms of bTB in the herd
While bTB is infectious, it rarely presents as a clinical disease in cattle. It is usually identified in animals that appear healthy but have positive reactions in diagnostic tuberculin tests.
Tuberculin is a purified protein derivative that is used to diagnose tuberculosis. bTB may be subacute or chronic, and progression of the disease can be highly variable. While a small number of animals may become severely ill and quickly display clinical symptoms, others may take several years to develop clinical symptoms. M. bovis can also lie dormant in hosts without causing disease.
Clinical symptoms of bTB include [1]:
- Weakness
- Loss of appetite
- Weight loss
- Fluctuating fever
- Dyspnea
- Intermittent hacking cough
- Low-grade pneumonia
- Diarrhea
- Prominent enlargement of the lymph nodes
Cows that test positive for bTB are usually culled. Infected cattle are rarely treated with antibiotics, because administering the required doses for long durations is not cost-effective.
The bacillus Calmette-Guérin (BCG) vaccine is available to people who are at risk of exposure to tuberculosis, but vaccination is not commonly used as a preventive measure for animals. This is due in part to a lack of safe and effective vaccines. Vaccination itself can interfere with bTB surveillance and diagnostic testing because it can lead to false-positive reactions to tuberculin. Research is underway to develop better bTB vaccines and to devise alternate routes of delivery for vaccinating wildlife.
Strategies for preventing bTB in your herd
Beef and dairy producers can help prevent the spread of bTB and reduce overall pathogen loads. The following biosecurity recommendations from the UK Department of Agriculture, Environment and Rural Affairs may help reduce risk [4].
By brought-in cattle
To prevent the introduction of bTB to your herd by bought-in cattle:
- Maintain a closed herd.
- Only purchase cattle directly from a known source.
- Avoid purchasing cattle that may have been moved frequently.
- Inquire about the origins of breeding cattle.
- Inquire about the bTB testing histories of animals you intend to purchase.
- If possible, quarantine cattle after purchase. Ask your veterinarian to conduct tuberculin testing on the animal(s) before allowing them to mix with other cattle.
- Separate purchased beef store/finishing cattle from your breeding stock.
By other animals and herds
To prevent the introduction of TB to your herd by badgers and deer
- Minimize direct and indirect contact between cattle, badgers, and deer.
- Do not allow cattle to graze in fields where badger setts (dens) are present or in areas where badgers or deer are active.
- Remove badger carcasses from fields.
- Do not overgraze fields.
- Fence off badger setts to prevent access by cattle.
- If possible, fence off badger paths and latrines.
- Design and manage troughs, drinkers, and mineral licks to make them less accessible to badgers.
- Do not allow badgers, deer, or other wildlife to access farm buildings, feed, feed stores, or silage pits.
Preventing the introduction of TB to your herd by cattle from other herds
- Maintain fences or boundaries that prevent contact with cattle from neighboring herds.
- Do not graze cattle in fields that are adjacent to cattle from neighboring herds.
- Do not force animals to share winter housing.
- Do not borrow bulls.
- Minimize the return of cattle from markets.
By people and equipment
Preventing the introduction of TB to your herd by people and equipment
- Minimize visitor contact with your herd.
- Ensure all visitors take precautions to prevent the introduction of bTB on your premises.
- Provide a disinfectant footbath.
- Clean and disinfect cattle housing and equipment before restocking.
- Change clothing and disinfect after visiting other herds and before coming into contact with your own cattle.
- Do not share equipment or vehicles with other farmers.
bTB diagnostic options
Diagnosing bTB can be complicated, because there is no universal strategy for diagnosing the disease in all infected animals. Clinical symptoms are not distinctive, and they make take many months to develop.
Diagnostic tests for bTB differ in terms of sensitivity and the stage of disease at which they will yield informative results (Figure 1). Serological tests for antibody or antigen detection and pathology examinations can be performed in the later stages of the disease. The most widely used diagnostics for early bTB detection are tuberculin skin tests and blood tests that measure the cytokine interferon gamma (IFN-γ), both of which provide insight into the cell-mediated immune response.
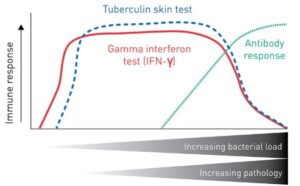
Tuberculin skin test
The tuberculin skin test is the standard tool for diagnosing TB in live domestic animals.
The test involves intradermal injection of a purified tuberculin extract derived from M. bovis. Skin thickness at the injection site is measured 72 hours later to detect swelling, which is a sign of delayed hypersensitivity associated with infection [1].
Interferon gamma blood test
An interferon gamma blood test is administered to measure IFN-γ released by blood cells in infected cattle after they are stimulated with bovine and avian tuberculins. The Applied Biosystems™ BOVIGAM™ IFN-γ assay is a highly effective test for early bTB detection, followed closely by the tuberculin skin test.
PCR as a confirmation tool
PCR is a reliable diagnostic tool for confirmation of the presence of mycobacteria that belong to the M. tuberculosis complex. PCR can return results much more quickly than tests based on bacterial culture. It can take eight weeks to obtain results for tests that require M. bovis culture, but a PCR workflow from sample preparation to testing can be completed in just three hours. The results are delivered to the farmer or veterinarian within a few days of testing.
Test selection
Test selection and implementation depend on the level of bTB risk in a given region and the goals of the specific bTB program.
An optimal bTB program allows sanitation decisions to be made quickly to expedite testing and culling and help minimize the duration of farm closures. With implementation of tuberculin skin testing and a BOVIGAM assay, a herd can be declared free of bTB in as few as four days.
The Applied Biosystems™ BOVIGAM™ TB Kit is the only in vitro bTB IFN-γ assay registered with the WOAH, and it has been validated by the WOAH as the primary stand-alone test for screening and confirmation of bTB in infected herds.
For more information about diagnostic solutions for bovine tuberculosis, visit our Bovine Tuberculosis Testing web page.
References
- Bovine Tuberculosis – WOAH – World Organization for Animal Health. WOAH – World Organization for Animal Health, 2023, https://www.woah.org/en/disease/bovine-tuberculosis/.
- Milián-Suazo F, González-Ruiz S, Contreras-Magallanes YG, et al. (2022) Vaccination strategies in a potential use of the vaccine against bovine tuberculosis in infected herds. Animals 12(23):3377 (https://doi.org/10.3390/ani122...).
- Skuce RA, Allen AR, McDowell SWJ (2012) Herd-level risk factors for bovine tuberculosis: a literature review. Vet Med Int 2012:1–10 (https://doi.org/10.1155/2012/6...).
- “What Is Bovine Tuberculosis (TB)? | Department of Agriculture, Environment and Rural Affairs”. DAERA, 2015, https://www.daera-ni.gov.uk/articles/what-bovine-tuberculosis-tb.
- Vordermeier M, Goodchild A, Clifton-Adley R, de la Rua R (2004) The interferon-gamma field trial: background, principles and progress. Vet Rec 155(2):37–38.
© For Veterinary Use Only. For In Vitro Use Only. Regulatory requirements vary by country; products may not be available in your geographic area. 2023 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified.



