



High Sperm Numbers - Detrimental Effects on Bull Sperm During Liquid Storage
Research from the University of Limerick looks at whether high sperm numbers have a detrimental effect on bull sperm during liquid storage.Introduction
Due to genomic selection, bulls which are in high
demand are now entering into artificial insemination
(AI) programmes at less than 1 year of age. A typical
ejaculate from these bulls will yield 50-150 frozen doses
compared to 500-1000 for a mature bull (20 x 106 sperm
per dose). To maximise the use of young elite sires
during a narrow breeding season it is imperative we
develop protocols for the use of liquid semen whereby
the sperm number per dose can be reduced without
affecting fertility. Currently in Ireland, liquid bull
semen contains 5 x 106 sperm per dose, compared to 2 x
106 sperm per dose in New Zealand (Verberckmoes et
al, 2005).
In addition, liquid semen has a limited
lifespan and is not used at greater than 2.5 days post
collection. The hypothesis of this study was that 5 x 106
sperm per dose is counter productive in terms of the
generation of oxidative stress, inhibition of sperm
mitochondrial activity as well as the consumption of
glucose within the diluent and therefore, reducing the
sperm number per dose will allow for liquid semen to be
stored successfully for longer duration.
Materials and Methods
Semen was collected from Holstein and British Friesian
bulls at a commercial AI centre (5 collections with 3-4
bulls per collection; collection = replicate). Sperm
concentration was assessed and the ejaculate was
diluted in a 5 per cent egg yolk Caprogen diluent (purged in N
gas) to a final concentration of 1 (T1), 2 (T2), 3 (T3), 4
(T4) and 5 (T5) x 106 sperm per insemination dose (0.25
mL straws). Semen from each bull was kept separate
and each bull was represented in each treatment.
Straws
were stored at ambient temperature and assessed in vitro
daily for 6 days (Day 0 = day of collection). On each
assessment day, semen was evaluated for progressive
linear motility (PLM), viability, oxidative stress,
mitochondrial activity and glucose consumption. PLM
was assessed using standard procedures. Sperm
viability, oxidative stress and mitochondrial activity
were examined using the fluorescent probes propidium
iodide (PI), CM-H2DCFDA and rhodamine 123 (R123),
respectively, and analysed using flow cytometry (BDLSR
1; BD Biosciences). Briefly, 1.4 x 106 sperm from
each treatment were washed twice in phosphate
buffered saline (PBS) at 800g for 10 min and incubated
at 37°C in the presence of 125 micrometre CM-H2DCFDA for
30 min followed by 300 micrometre PI for 15 min.
A final wash
was performed in PBS to remove excess stain and
10,000 events were analysed. Mitochondrial activity
was analysed as above with the exception of a 15 min
incubation at 37°C with 2.5 micrometre R123. For glucose
analysis samples were centrifuged at 8000g at 4°C and
the supernatant was removed, frozen at -20°C and
analysed later using a commercial glucose assay kit
(Megazyme, Ireland). Briefly, samples were diluted 1:2
with distilled water, added to 1 mL of glucose oxidase/peroxidase reagent and incubated at 45°C for 20
min.
Absorbance was measured on a spectrophotometer
and concentrations of glucose were determined using a
standard curve. Data were examined for normality,
transformed where appropriate and analysed using
repeated measures in SPSS (version 20.0). The model
included the main effects of day, treatment and day x
treatment interactions. Results are presented as mean ±
s.e.m.
Results and Discussion
There was no treatment x day interaction for any of the
variables assessed. PLM declined with duration of
storage from 95.1 ± 0.68 per cent to 73.8 ± 0.99 per cent for all
treatments from Day 0 to 5, respectively (P<0.001).
There was an effect of day and treatment on percentage
live (P<0.001) with the highest percentage live in T1
and the lowest in T5 on all days (Day 0: 92.4 ± 0.74 and
67.0 ± 2.96 per cent for T1 and T5, respectively, vs. Day 5:
82.8 ± 2.20 and 58.8 ± 3.56 per cent for T1 and T5,
respectively. The percentage of live sperm positive for
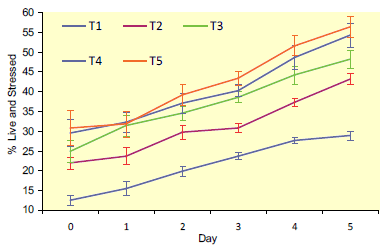
CM-H2DCFDA increased in all treatments on each day
of storage (Fig. 1; P<0.001).
There was an effect of
treatment (P<0.001) with T1 having the lowest
percentage of live sperm positive for CM-H2DCFDA
every day and T5 the highest on five days. The
percentage of live sperm positive for R123 ranged
between 91.6 and 95.8 per cent and was not affected by
treatment (P>0.05). The level of glucose in Caprogen
declined with duration of storage (P<0.001), being
lowest in T5 and highest in T1 on Day 5 (808.6 ± 23.90
and 964.6 ± 18.90 micrograms/mL, respectively).
Conclusions
Reducing the sperm number per dose reduces the level of oxidative stress and may be beneficial to the prolonged storage of liquid bull semen.
Acknowledgments
We gratefully acknowledge support from IRCSET and Enterprise Ireland. Semen was donated by the National Cattle Breeding Centre, Enfield, Co. Meath.
Reference
Verberckmoes, S., Van Soom, A., Dewulf, J., de Kruif, A. (2005) Theriogenology 63: 912–922
May 2012

