



Microbial Risks In Feeding Colostrum To Calves
Microbial contamination of colostrum can contribute to calfhood disease and can interfere with passive absorption of colostral antibodies, producers should adopt management strategies to reduce bacterial counts in colostrum fed to calves. S Godden from the Department of Veterinary Population Medicine at University of Minnesota looks at minising the risks.Preventing bacterial contamination during colostrum harvest or feeding
Methods to avoid pathogen contamination from infected glands or fecal contamination include preventing the calf from suckling the dam, careful attention to the udder preparation routine prior to harvesting colostrum and avoiding pooling of raw colostrum. It may also be useful to identify infected cows, an example being to test cows for infection with MAP prior to calving. However, given the poor sensitivity of diagnostic tests for MAP, the latter approach is likely to be imperfect. Development and strict adherence to protocols for cleaning and sanitation of milking, Stewart et al., (2005) emphasised the importance of udder preparation, equipment sanitation, and proper storage techniques in order to prevent bacterial contamination and proliferation in fresh colostrum.
The first objective of this study was to identify control points for bacterial contamination of colostrum during the harvest and feeding processes. First-milking colostrum samples were collected aseptically directly from the mammary gland of 39 cows, from the milking bucket, and from the esophageal feeder tube. All samples underwent bacteriological culture for total plate count and total coliform count. Bacteria counts were generally low or nil in colostrum collected directly from the gland (geometric meanudder = 27.5 cfu/ml).
However, significant bacterial contamination occurred during the process of milking the colostrum into the bucket (geometric meanbucket = 97,724 cfu/ml). No additional bacterial contamination occurred between the bucket and the esophageal feeder tube. These results emphasise the importance of properly prepping and sanitising udders prior to colostrum harvest, milking into a clean, sanitised bucket, and transferring colostrum into clean, sanitised storage or feeding equipment (Stewart et al., 2005).
Preventing bacterial proliferation in stored colostrum
It is well understood that bacteria present in stored colostrum or milk can begin to multiply rapidly if stored at warm ambient temperatures, but will still multiply, albeit more slowly, in the refrigerator. If colostrum is not to be fed within 1-2 hours of collection, it should be quickly refrigerated (for up to 48 hours) or frozen. Use of potassium sorbate preservative may also delay bacterial proliferation in refrigerated colostrum. This effect was amply demonstrated by Stewart et al., (2005) with the completion of a second study to describe the effect of refrigeration (vs ambient temperature) and use of potassium sorbate preservative (vs no preservative) on bacteria counts in stored fresh colostrum. For this study aliquots of colostrum were collected from the milking bucket and allocated to one of four treatment groups: I) Refrigeration (approx. 40 °Fahrenheit) , 2) Ambient temperature (approx. 73 °Fahrenheit) , 3) Refrigeration with potassium sorbate preservative (0.5 per cent solution) and 4) Ambient temperature with potassium sorbate preservative.
Subsamples from each treatment group were collected after 0, 24, 48, and 96 h of storage. Storing colostrum at warm ambient temperatures resulted in the most rapid increase in bacteria counts, followed by intermediate rates of growth in non-preserved refrigerated samples or preserved samples stored at ambient temperature. However, by 48 hours of age, bacteria counts in refrigerated, non-preserved samples (group i) or preserved ambient temperature samples (group iv), where just as high as for non-preserved samples stored at ambient temperature (group ii). The most effective treatment studied was the use of potassium sorbate preservative in refrigerated samples (group iii), for which total plate count and total coliform counts dropped significantly and then remained constant during the 96-h storage period (Figure 1).
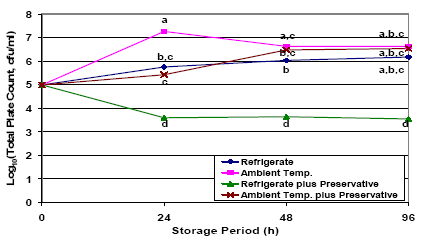
The results of this research suggest that, at a minimum, producers should refrigerate colostrum as quickly as possible after collection, if it is to be stored for more than a couple of hours before feeding. These results also suggest that producers should try to feed up non-preserved stored colostrum as rapidly as possible (goal < 2 days). Though not widely adopted by the industry, these results show the benefits of combining the use of a preservative (approximate cost = $0.50 per gallon) to prevent bacterial proliferation in stored colostrum for at least as long as 96 hours. Studies describing the final shelf-life of preserved colostrum are ongoing. Information on potassium sorbate suppliers, mixing and use can be found at: http://www.atticacows.com/orgMain.asp?orgid=19&storyTypeID=&sid=&. While the use of preservatives looks promising, further research on preservatives is needed, as some preservatives may damage colostral immunoglobulins.
All producers should take steps to minimise contamination or bacterial proliferation during harvest, storage, or feeding. Additional steps producers may consider include discarding colostrum from high risk or known infected cows (e.g. Johne¡¯s test-positive cows) (McGuirk et al., 2004). Producers should also avoid pooling fresh colostrum, as this may increase the risk of transmitting infectious pathogens to more than one calf. Freezing colostrum is one additional method to prevent bacterial proliferation in stored colostrum. However, producers must be cautious not to overheat colostrum during the thawing process (keep ¡Ü 140 ¡ãF) or IgG denaturation could occur. Additional tools that some producers may consider using include the use of commercial colostrum replacers or feeding pasteurised colostrum.
Additional tools designed to reduce or eliminate pathogen exposure through colostrum include the feeding commercial colostrum replacers or pasteurising colostrum. These two options will be discussed next.
Commercial colostrum replacers
Commercial colostrum replacement (CR) products may provide a viable alternative to feeding maternal colostrum and could serve as a very effective management tool to prevent colostral disease transmission. These CR products contain bovine Ig that is typically either lacteal-derived or plasma-derived and are intended to completely replace maternal colostrum feedings. The CR should contain a minimum of 100 grams of IgG per dose, the minimum recommended dose in order for calves to receive to attain a predicted final serum IgG > 10 mg/ml (Quigley et al., 2001; Quigley et al., 2002), and must also contain a nutrient pack that provides a source of protein, energy, vitamins and minerals similar to levels found in maternal colostrum. If CR products prove to be an effective substitute for maternal colostrum while potentially reducing disease transmission, they could serve as one critical control point for preventing the transmission of several infectious diseases, including Johne’s disease (Mycobacterium avium subsp. paratuberculosis, MAP). These products have the added benefit of being convenient to quickly mix and feed.
In a recent controlled field study of 12 Midwest dairy farms initiated in 2003, heifer calves were separated from their dams within 0.5 to 1 h after birth and systematically assigned (alternately for every other calf born) to be fed maternal colostrum (MC, n = 261) or colostrum replacer (CR, n = 236). The heifer calves were followed to adulthood and tested for MAP infection using a commercially available ELISA assay and the conventional bacterial fecal culture test for MAP at 30, 42, and 54 months of age. Results showed that calves fed the CR had an estimated 44 per cent reduction in risk for testing positive to MAP (ELISA and /or Fecal culture) as compared with calves fed MC at birth (Haz. ratio = 0.559, P = 0.056) (Pithua et al., 2009). This study demonstrated that raw maternal colostrum can be an important source for transmission of MAP to newborn calves, and showed that colostrum replacement products can be an effective management tool in infected dairy herds that are attempting to reduce the prevalence of Johne’s disease.
Despite their potential to control transmission of some diseases, the results of early CR product research has shown mixed results in their ability to consistently achieve successful passive transfer in calves (serum IgG < 10.0 mg/ml) (Quigley et al., 2001). However, studies seem to report better rates of successful passive transfer (serum IgG > 10.0 mg/ml) when calves were fed higher doses (IgG mass) in a CR product. This led Quigley to suggest feeding higher doses of CR, thereby increasing the IgG intake and improving the 24 hour serum IgG concentrations of calves. One example of this: Jones et al (2004) reported an average serum IgG concentration of 13.96 mg/ml in calves fed two doses of a CR product in two feedings (total dose = 249 g IgG for Holsteins or 186 g IgG for Jerseys). More recently a study reported that the average 24 hr serum IgG level for calves fed either 1 dose (100 g IgG) or 2 doses (200 g IgG) of a lacteal-derived commercially available colostrum-derived product were 11.6 ± 2.9 mg/ml and 16.9 (± 6.2) mg/ml, respectively (Land O’ Lakes Colostrum Replacement. Land O’ Lakes Inc. St. Paul, MN) (Foster et al., 2006). In a similarly designed study using the same commercial CR product, the average serum IgG for calves fed either 1 dose (100 g IgG) or doses (200 g IgG) was 9.6 mg/ml and 19.0 mg/ml, respectively. In this second study, feeding 2 doses (200 g IgG) of this CR product produced serum IgG levels similar to feeding 4 quarts of fresh maternal colostrum (20.7 mg/ml) (Godden et al., 2009).
In summary, CR products may offer producers a convenient way to provide adequate passive immunity to dairy calves while reducing the risk of pathogen exposure through colostrum. Feeding CR products is certainly recommended in situations where a sufficient volume of clean, high quality colostrum is not available from the cow and when stored colostrum is not available. Large scale, long-term studies are still needed to describe the health and economic-benefit of adopting this practice as a routine management tool. If using colostrum replacers, producers are advised to feed 150 to 200 g IgG in a colostrum replacer product that has been previously tested for efficacy.
General recommendations for on-farm pasteurisation of colostrum.
Handling of raw and pasteurised colostrum:- Minimise contamination of raw colostrum by collecting colostrum from a properly prepped, disinfected udder into a clean sanitised bucket.
- If there is to be greater than a 2 hour delay between colostrum collection and pasteurisation, refrigerate the raw colostrum in sanitised covered containers.
- After pasteurisation is completed, quickly cool colostrum and then either feed to calves within two hours, refrigerate in covered sanitised containers, or freeze in clean containers or bags. This is to prevent recontamination and to delay or slow the regrowth of bacteria.
- Ensure proper cleaning and sanitation of the pasteuriser equipment plus colostrum collection, storage and feeding equipment.
- Use a batch pasteuriser design (not HTST).
- Pasteurise at 140 °F (60 °C) for 60 minutes. Do not allow temperatures to fluctuate above 141 °C or denaturation of IgG will begin to occur.
- Agitate colostrum continuously during the heating, pasteurisation, and cooling processes.
- Routinely monitor times and temperatures during the pasteurisation cycle.
- Record and monitor health records. Goals for preweaning treatment and mortality rates are < 25 per cent and < 5 per cent, respectively (McGuirk and Collins, 2004). Note: this monitoring should be done for all operations, even if pasteurised colostrum is not fed on the dairy.
- Monitor passive transfer of immunity. An excellent way to do this is to use a hand-held refractometer to measure serum total protein levels. This should be done in 12 or more clinically normal calves between 1 to 7 days of age. The goal is for ¡Ý 90 per cent of calves tested to have a serum TP value ¡Ý 5.0 gm/dl. Note: This monitoring is encouraged for all operations, even if pasteurised colostrum is not fed on the dairy.
- Periodic culture of raw and heat-treated colostrum samples to monitor efficacy of the heat-treatment process (Goal: Total Bacteria Count in pasteurised colostrum < 20,000 cfu/ml). Paired frozen colostrum samples can be sent to a microbiology lab for this. You must request that lab technicians to be prepared to do multiple dilutions of colostrum.
Conclusion
Because microbial contamination of colostrum can contribute to calfhood disease and can interfere with passive absorption of colostral antibodies, producers should adopt management strategies to reduce bacterial counts in colostrum fed to calves. All producers should pay attention to hygiene and sanitation so as to minimise bacterial contamination during the colostrum harvest, storage and feeding processes. Producers should also take steps to avoid bacterial proliferation if storing colostrum. Methods to achieve this could include rapid refrigeration, freezing, rapid turnover of fresh colostrum (< 48 hrs) and possible use of chemical preservatives. Additional management tools that could further reduce pathogen exposure through colostrum can include feeding commercial colostrum replacement products or feeding pasteurised colostrum.
June 2010

