



Detecting Carcass Contamination with Spectroscopic Imaging
A preliminary investigation analysed markers of faecal contamination in cattle diets in a bid to improve meat safety, says M.R.F. Lee, V.J. Theobald, M. K. Theodorou, Veberg Dahl, F. Lundby and J. P. Wold. This paper was from the international conference Advancing Beef Safety through Research and Innovation organised by ProSafeBeef - a European Commission Research Project.Beef production within the EU region is an activity of major economic importance, valued at €75 billion. However, reforms to the Common Agricultural Policy, increased globalisation, reduced commodity prices and an increasingly sophisticated, health-conscious consumer are requiring the industry to produce beef and beef products that are convenient, traceable, nutritious and of consistent quality.
Alongside these considerations, today’s consumer demands assurances regarding food safety and health, which is of paramount importance given the serious impact of beef related health scares. In order to boost consumer trust and invigorate the industry, a Framework 6 European Union Project - ProSafeBeef is examining new ways of reducing contaminants in the beef chain from ‘farm to fork’ as well as enhancing quality, choice and diversity.
Cleanliness in the abattoir is of the utmost importance and numerous practices are carried out on farm to ensure that the animals arrive at the abattoir with limited faecal matter clinging to the hide.
Such strategies include: change to hay and cereal based diets before slaughter to encourage ‘dry’ faeces; cleaning the animals before they travel; reducing stress on the animals during transport and at the abattoir to limit pathogen shedding. However, even with these strategies in place, a major source of contamination in the abattoir is from small traces of faeces still associated with the hide coming into contact with the dressed carcass.
Currently carcasses are checked by ‘eye’ and washed with chemical sprays or dissected to remove contaminated areas. Unfortunately small areas of faecal contamination may not be visible to the eye and may harbour millions of pathogenic bacteria. Spectroscopic imaging is a rapidly evolving research area, with the potential to provide real-time solutions for the detection of faecal contamination on carcasses. Chlorophyll is ubiquitous in green plants and thus livestock diets.
During digestion in the gut, chlorophyll is only partially degraded to coloured and fluorescent intermediates: the phaeophytin, chlorophyllide, phaeophorbide and pyrophaeophorbide derivatives of chlorophylls a and b.
In a preliminary investigation we investigated the best chlorophyll breakdown products for use as markers of faecal contamination on a range of diets. Eight Cheviot sheep were fed either:
- fresh grass and clover,
- grass silage,
- hay, or
- concentrate and barley straw.
Each diet was offered to two sheep for a period of two weeks before diet change over in a duplicate 4 x 4 Latin Square design. Samples of feed were collected during the whole feeding period and bulked whilst samples of faeces were taken at the end of the period.
Samples were measured for chlorophyll and its derivatives using HPLC. Fluorescence emission spectra were measured directly on the faeces. The samples were placed into sample cuvettes, which exposed a flat circular surface with a diameter of 5 cm for the measurements. The fluorescence emission spectra were measured with excitation at 382 and 430 nm, using an optical bench system, suitable for solutions and solid samples. The excitation light was generated by a 300 W Xenon light source (Oriel 6258, Oriel Corporation, Stratford, CT) and passed through a 10 nm bandwidth interference filter (Oriel 59920) and (Oriel 59295). The light was directed onto the samples at an angle of about 45°.
The spectra were collected by an imaging spectrograph (Acton SP-150, Acton Research Corporation, Acton, MA) connected to a sensitive charge coupled device (CCD-camera) (Roper Scientific NTE/CCD-1340/400-EMB, Roper Scientific, Trenton, NJ). Cut-off filters at 400 nm (for the 382 nm excitation) (Melles Griot 03FCG049) and 475 nm (for the 430 nm excitation) (Melles Griot 03FCG068) were positioned in front of the spectrograph slit to suppress excitation light reflected from the samples.
Exposure time was 10 and 5 sec for excitation at 382 and 430 nm, respectively. The temperature of the samples was 4 °C. All the samples were measured twice and an average was used in the analysis. The field of illumination was not perfectly homogenous, so the samples were rotated 90° between each measurement to even out sample heterogeneity. To ensure stable illumination, the emission intensity at 440 nm at excitation 382 nm was measured from a stable fluorescence standard of washable plastic (Ciba, Basel, Switzerland) before and after the measurements.
Fluorescence images were collected with the same system, slightly modified. A Nikon 102 mm photographic lens was mounted on the imaging spectrograph, the spectrograph slit was removed and the grating was exchanged with a mirror. Spectral images were created by placing a 40 nm bandwidth interference filter in front of the lens. Samples were illuminated by 400 nm (10 nm bandwidth filter, Melles Griot 03FIV026) light and images were captured at 685 nm (10 nm bandwidth). Exposure time for each image was 60s.
Not surprisingly animals offered the fresh grass and clover diets had a greater concentration of fluorescent compounds in their faeces and subsequent fluorescence than animals on conserved forages and on concentrate based diets. Consequently the accuracy of the spectral imaging detection of faecal contamination depended on the diet of the animal. This is why previous similar techniques such as ‘Verifeye®’ have not been universally accepted. In a second experiment we investigated natural markers which can be added to the diet before slaughter which will increase the intensity of the fluorescence.
Ten Cheviot sheep were offered a concentrate and barley diet and split into five treatment groups during a duplicate 5 x 5 Latin square design where each period lasted 2 weeks. Four of the groups received a different chlorophyll based marker at a rate of 1 g per day for the second week of the experimental period. The last group received no supplement and was used as the control. At the end of each period faeces were collected to be analysed as in the first experiment (HPLC and fluorescence) before the animals changed treatment.
The results are shown in figure 1 where each of the markers significantly increased the fluorescence intensity of the faeces over the control. The use of markers in pre-slaughter diets would thus improve the accuracy of faecal detection as a result of greater fluorescence and pin pointing the excitation wavelengths of the marker to help with visualisation. Further work is being continued to identify the most suitable marker and feeding regime to supply the marker.
Figure 1
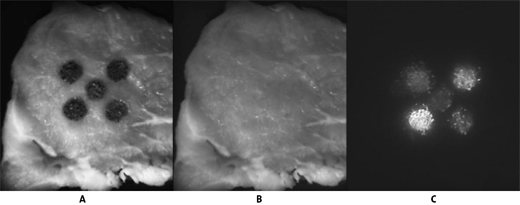
Picture A – Meat sample with five spots of faeces from animals on a concentrate diet. The central spot is the control where animals were not fed a marker the surrounding spots animals supplemented with four different markers for 1 week.
Picture B – The faeces were washed off the meat with water.
Picture C – Is the same as Picture B but under ultra violet light showing the potential of two of the markers. Note the control sample can hardly be seen.
Further Reading
| - | You can view all the conference papers of Advancing Beef Safety through Research and Innovation by clicking here. |
May 2009



