



US Concerns Raised over EU Stance on GM Food and Feed
While the European agricultural industry is a heavy consumer of genetically modified products - largely in animal feed - the industry is restricted in what it can grow, writes Chris Harris, TheCattleSite senior editor.Directives from the EU allow just one kind of GM maize to be grown in European states and no genetically modified soybean.
The lack of home grown genetically modified grains and soybean meal for animal feed means that the EU is a large importer of the products, largely from North and South America.
For while the products cannot be grown, they can be and are used in feed and food in large proportions.
However, a recent report by the USDA Foreign Agricultural Service, says that the EU farming community has benefited from genetically engineered crops through higher yields and cost savings.
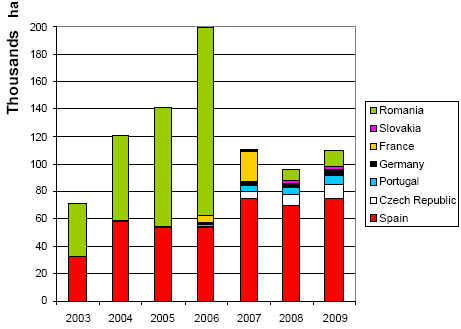
It says that despite being bound by strict regulations - that at present only allow genetically modified maize to be grown - and political threats from lobby groups, the area that is devoted to growing biotech corn is expected to increase next year, reaching 110,000 hectares.
"Since the approval of the first biotech corn event for planting in the EU, Spain has been the country that has most rapidly adopted its use," the report says.
"Prior to its accession to the EU, Romania was a major producer of biotech soybeans. However, since biotech soybeans are not approved for planting in the EU, this ended in 2007 with its accession to the EU."
The report, EU-27 Biotechnology Annual Report 2008, also shows that despite the fact that genetically modifies soybean meal is not produced in the EU, it is the largest GM product used in the EU.
The EU-27 consumes about 33 million tonnes of soybean meal a year in animal feed, with most of it imported from North and South America. Of this between 80 and 95 per cent is genetically modified - 26 million to 31 million tonnes.
There is also a niche market for non-genetically modified soybeans and soybean meal used in animal feed, mainly for the poultry sector and other animal production mainly under the Identity Preservation programme or geographical indications, or for human consumption for soybeans, such as in baby food.
The amount of genetically modified corn that is used is small by comparison with just 10 to 25 per cent of the total consumed being GM.
The European Union on the other hand says that it has put in place its stringent regulations on genetically engineered foods to protect animal health and the environment and to ensure the free and safe movement of healthy genetically modified products within the EU.
The European Commission explains in its guidance notes on genetically modified organisms that the regulations are there to protect consumers.
The commission says: "The Regulation stipulates that the products to which it applies must not:
- have adverse effects on human health, animal health, or the environment;
- mislead the consumer or user;
- differ from the food/feed they are intended to replace to such an extent that their normal consumption would be nutritionally disadvantageous for human beings (and for animals in the case of genetically modified feed).
- in the case of genetically modified food and feed, harm or mislead the consumer by impairing the distinctive features of the animal products.
"The Regulation puts in place a centralised, uniform and transparent EU procedure for all applications for placing on the market, whether they concern the GMO itself or the food and feed products derived therefrom."
The commission says that several genetically modified organisms have been authorised as products containing GMOs or consisting of these organisms for use in feed. These are chiefly maize varieties, rape varieties and one soya variety.
The restrictions for genetically modified foods are also tightened by the labelling regulations set out by the commission.
The FAS report says the regulations are an attempt to address the problem of unintentional contamination of non-genetically modified foods and feed with genetically engineered products.
"As long as the GM-derived food ingredient material was below one per cent of individual ingredients, food stuffs would not be subject to specific labelling requirements," the report says.
It adds that the labelling and traceability regulations seek to establish a greater coherence in the regulatory framework.
The European Commission explains that the regulations are consistent for food and feed.
"Genetically modified foods which are delivered as such to the final consumer or mass caterers (restaurants, hospitals, canteens and similar caterers) must be labelled in accordance with Article 12 of Regulation (EC) No 1829/2003, regardless of whether DNA or proteins derived from genetic modification are contained in the final product or not," it says.
"The labelling requirement also includes highly refined products, such as oil obtained from genetically modified maize.
"The same rules apply to animal feed, including any compound feed that contains transgenic soya. Corn gluten feed produced from transgenic maize must also be labelled, in compliance with Article 25 of Regulation (EC) No 1829/2003, so as to provide livestock farmers with accurate information on the composition and properties of feed.
"Therefore, genetically modified food and feed are subject to the specific labelling requirements imposed by the GMO legislation.
"However, besides these specific labelling requirements, genetically modified food is subject to the labelling requirements of the general legislation in this area."
The Commission adds that although the animal feed has to be labelled, it does not affect the meat, eggs or milk produced by the animals given the modified feed.
"In line with the general EU rules on labelling, Regulation (EC) No 1829/2003 does not require labelling of products such as meat, milk or eggs obtained from animals fed with genetically modified feed or treated with genetically modified medicinal products. Nor are these products subject to traceability requirements," the Commission says.
However, the FAS says that this regulatory approach to biotechnology by the EU has a significant impact on international trade.
The stance has even led to the EU being taken through the World Trade Organisation disputes procedure.
"The European Commission and the United States have a continuing dialogue on how to normalize trade in products of modern agricultural biotechnology. This dialogue is an effort to address and correct the WTO inconsistent parts of the EU's process," the FAS says.
It adds that the EU is facing serious challenges over its stance on genetically modified products and new agricultural biotechnology.
As the science of biotechnology advances, the FAS says the EU has been facing similar choices over labelling and traceability and issues of public perception that it was initially over genetically engineered products.
The European Commission recently proposed a new novel foods regulation, which includes coverage of food products from cloning and nanotechnology.
However, the FAS has criticised the European Commission's stance.
"In its current form, many key components of this proposal are ill-defined. In addition, it envisions an onerous pre-market approval process. It could also require products approved under the regulation to carry mandatory labels and to be subject to significant post-market monitoring, even if determined to be substantially equivalent to conventional counterparts," it says in the report.
These concerns are now being directed toward the EU's stance on food products from cloned animals.
At present there are none of these products on the EU market but an opinion from the European Food Safety Agency at the end of last year and a report in July this year showed that there was no evidence that the products from cloned animals would pose any greater risk than those from naturally bred animals.
"In the draft report EFSA stated that researchers found no difference exceeding the normal variability in the composition and nutritional value of meat from swine and cattle and cow milk between healthy clones or the progeny of clones and their conventional counterparts. The currently available data indicate that food products from cloned cattle and pigs and their progeny are as safe as food products of livestock derived by conventional breeding," the DFAS report says.
However, it adds that a report from the European Group of Ethics cast doubts on whether cloning animals for food supply is ethically justified.
Further Reading
| - | You can view the full EU 27 Biotechnology Annual Report by clicking here. |
December 2008


